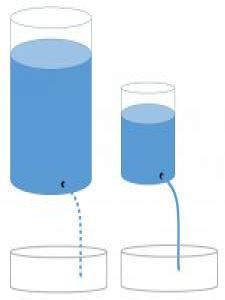
Engage: Go with the flow?
A chemistry teacher uses a model similar to the one pictured above to represent the concept of differences in heat capacity. The teacher asks the class to imagine that the water represents heat and to predict how the flow of water might relate to heat capacity, temperature, and thermal conductivity. The model includes a large container with a slow leak and a small container with a fast leak. The teachers asks "Which of the two leaking containers could produce the largest water level change in identical pans placed below the streams of water?" This is what two friends in class said:
Kelvin: The water in the smaller container will cause the water level to change more because is has a faster flow.
Joule: The water in the larger container will cause the water level to change more because it holds the most water.
With whom do you agree and why? Record your thoughts in your science notebook.
Explore: Where does the heat go?
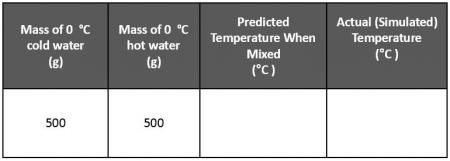
Everybody knows that when you mix hot and cold water, you get warm water. Can you predict the temperature of the warm mixture if you mix 500 g of 100 °C water and 500 g of 0 °C water? Create a data table in your notebook similar to the one below and record your prediction.
What if we change the 100 °C water to another 100 °C liquid? Will the heat transfer cause the same temperature change? Why or why not? Record your thoughts in your science notebook. You may use a data table similar to the one below or create your own.
| Mass of 0 °C Water (g) | Mass of 100 °C Liquid X (g) |
Predicted Temperature When Mixed (°C) |
Actual (Simulated) Temperature (°C) |
|---|---|---|---|
|
500
|
500
|
Explain 1: Equal temperature, equal heat?
In the mini-investigation you saw that even though the 100 °C water and the 100 °C liquid X were at the same temperature, the final temperature when mixed with 0 °C water was different in each case. Does equal temperature mean equal heat transfer? Record your answer to the question in your science notebook based on what you learn in the video.
Explain 2: What’s so specific about heat capacity?
What’s the difference between heat capacity and specific heat capacity? View the video and answer the question in your science notebook. You may stop the video at 4:16.
Elaborate: How much heat does it take?
The amount of heat required to increase the temperature of a pure substance depends on a few variables. Do you know which ones they are? In your science notebook, write down all of the variables in the list below that you think might apply.
- Density
- Mass
- Molarity
- Pressure
- pH
- Specific heat capacity
- Temperature
Watch the video to check your answer (0:00–5:09), and then solve the problem in your science notebook along with your video host (5:09–7:02).
Problem: How much heat is required to raise the temperature of 250 g of liquid water by 47 °C?
Now that you have explored the concept of specific heat capacity and have been introduced to how it’s used to solve problems, you are ready for the calculations you will do in your chemistry class. But, before you go, check your concept knowledge in the next section.
Evaluate: Concept Check
At the beginning of this lesson, you were shown a diagram of water leaking from two different containers. Now that you have finished the lesson, review what you wrote in the Engage in order to answer these questions—
- What does the water in the container represent?
- What does the flow of water represent?
- What does the final water level in the pans represent?
- What does the size of the containers represent?
Submit your answers in the following check for understanding.
Teacher Notes
This resource is a curated collection of interactives, videos, and other digital media assembled in a conceptually scaffolded 5E lesson format. It provides alternative or additional tier-one learning options for students learning about the concept of specific heat, which supports Chemistry TEKS (11)(D). The assignments require student participation with self-checked and teacher-checked formative assessment opportunities. For example, after students record observations and data in their notebooks, they may be prompted to be prepared to share their answers with the class.
Check for prerequisite knowledge, differentiation needs, and student follow-up requirements (as necessary) by reviewing the resource before assigning it to or working through it with your students.
Critical Vocabulary
- Heat
- Temperature
- Specific Heat Capacity
Resource Map
How to Use this Resource
This resource can be used for instruction in a variety of ways.
- Use with a single computer and projector; this can be delivered in a traditional classroom.
- Use with a combination of individual student computers and teacher computer and projector (in either a computer lab or other 1:1 environment).
- Assign the resource to students as work to do outside of the school day as part of a flipped classroom to allow application, practice, and additional support during the school day.
- Use with students as tutorials.
- Share with parents to inform them about what their child is learning in school.
- Use with students who are unable to participate in the traditional classroom environment.